Determination of Sodium by Flame Emission Spectroscopy
Potts in Treatise on Geochemistry Second Edition 2014 1592 Some History. Atomic emission spectroscopy ICP-AESPES is used in.
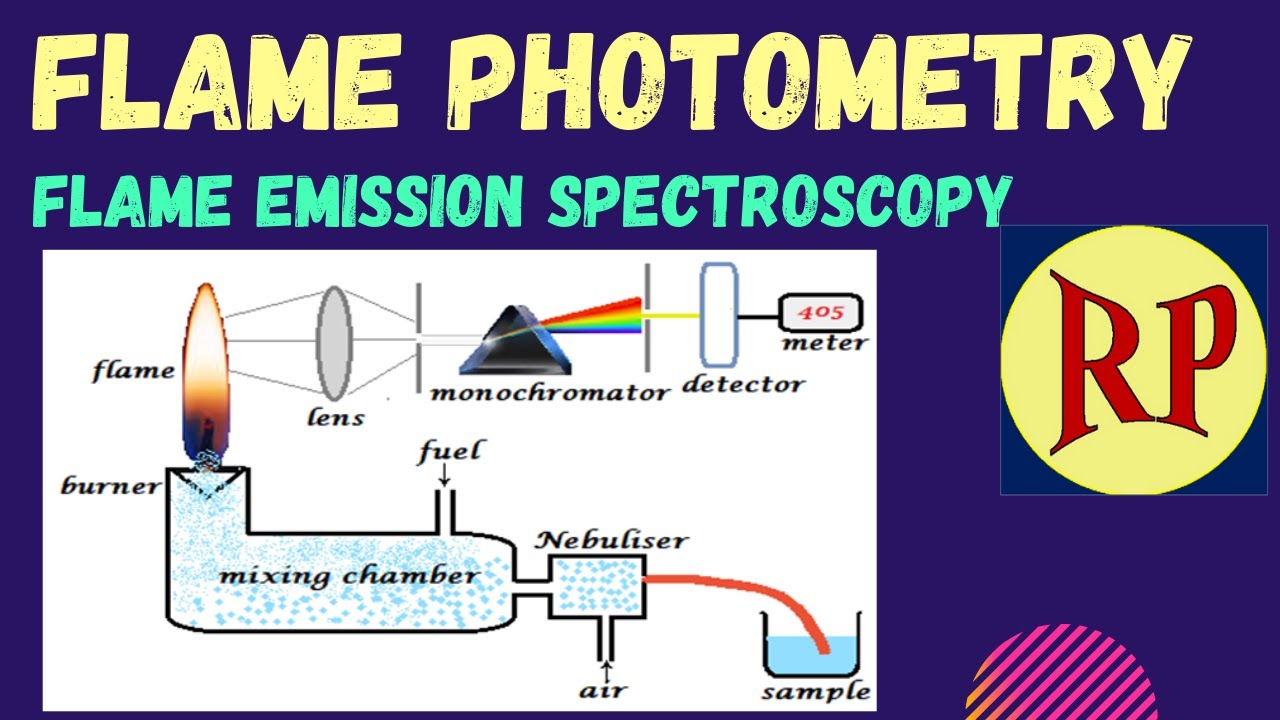
Flame Photometry Flame Emission Spectroscopy Fes Atomic Emission Spectroscopy Aes Youtube
Analysis of Ash and Minerals.
. Determination of total arsenic content in water by atomic absorption spectroscopy AAS using vapour generation assembly VGA. The ash content is a measure of the total amount of minerals present within a food whereas the mineral content is a measure of the amount of specific inorganic components present within a food such as Ca Na K and ClDetermination of the ash and mineral content of foods is important for a number of reasons. ASTM International formerly the American Society for.
Aqueous film forming foam. The detection of metal deficiency in soils and plants. Flame photometry is one of the branches of atomic absorption spectroscopy.
Atomic spectroscopy has seen a range of influential discoveries and developments over the last 400 years and a fascinating time line of these developments has been published by Thomsen 2006Arguably the key scientific discovery that established optical atomic spectrometry was. Recommended Beer Degassing Methods and Alternatives Matrix. 2-Amino-3-5-methyl-3-oxo-12- oxazol-4-yl propanoic acid.
The analysis of agricultural materials. It has been used for thousands of applications involving a wide diversity of samples. Which of the following spectroscopy techniques is associated with molecular emission.
What are the ten uses of atomic emission spectroscopy. Association of Analytical Chemists. Clinical diagnosis of different metals ie Na K Ca Mg etc in body fluids.
MALDI-QTOF MS Matrix-assisted Laser DesorptionIonization Time of Flight Mass Spectroscopy has been used to quantify vegetable oil in milk Garcia Sanvido Saraiva. A UV-Visible spectroscopy B IR spectroscopy C Fluorescence spectroscopy D X-ray diffraction. Also known as.
In analytical chemistry the. Atomic emission spectroscopy AES is a method of chemical analysis that uses the intensity of light emitted from a flame plasma arc or spark at a particular wavelength to determine the quantity of an element in a sample. Gamma spectroscopy is a radionuclide measurement method.
The determination of trace metals in solution. Atomic absorption spectrometry AAS is a very sensitive method of elemental analysis allowing the determination of metals in a variety of samples at the picogram level. Data Science for Advancing Environmental Science Engineering and Technology.
Butcher in Encyclopedia of Analytical Science Second Edition 2005 Introduction. The application of flame atomic absorption spectrometry for gold determination in some of its bearing rocks. Multiple samples release of volatiles can negatively impact.
Presence of urine has also been reported by observing change in the concentration of sodium and calcium in samples undergoing flame atomic absorption spectroscopy Santos et al. Atomic absorption spectroscopy AAS and atomic emission spectroscopy AES are Spectro analytical procedures that use the absorption of optical radiation light by free atoms in the gaseous state to determine chemical elements quantitatively. Behari JR Prakash R.
Diffraction gratings work based on A Max-Well Boltzmanns equation B Braggs equation C Noise-Whitney equation D Beers law. American Public Health Association. While a Geiger counter determines only the count rate a gamma spectrometer will determine the energy and the count rate of gamma-rays emitted by radioactive substances.
The wavelength of the atomic spectral line in the emission spectrum gives the identity of the element while the intensity of the emitted light is proportional. Atomic absorption spectroscopy AAS and atomic emission spectroscopy AES is a spectroanalytical procedure for the quantitative determination of chemical elements using the absorption of optical radiation light by free atoms in the gaseous stateAtomic absorption spectroscopy is based on absorption of light by free metallic ions. The absorption of light by free metallic ions is the basis for atomic absorption spectroscopy.
Call for Papers Special Issue. Flame photometer can be used to determine the concentration of certain metal ions like sodium potassium lithium calcium and cesium etc. It is also known as flame emission spectroscopy.
Some of the important types of Spectroscopic Techniques are as follows. Atomic emission spectroscopy. This joint call for papers by EST and EST Letters seeks contributions on Machine Learning and Artificial Intelligence research that investigates supports and advances our understanding of natural and engineered environmental systemsThe deadline for.
The degassing of beer is a critical sample-preparation step for many beer analyses. Currently it has become a necessary tool in the field of analytical chemistry. Summary of Method 21 In direct aspiration atomic absorption spectroscopy a sample is aspirated and atomized in a flame A light beam from a hollow cathode lamp whose cathode is made of the element to be determined is directed through the flame into a monochromator and onto a detector that measures the amount of light absorbed Absorption depends upon the.
Although multiple options exist for degassing each has its own advantages and disadvantages including degassing time cost throughput one sample vs. Atomic fluorescence spectroscopy.

Flame Photometry An Overview Sciencedirect Topics

Emission Spectra And Photographs From The Flame Tests Of Lithium Download Scientific Diagram

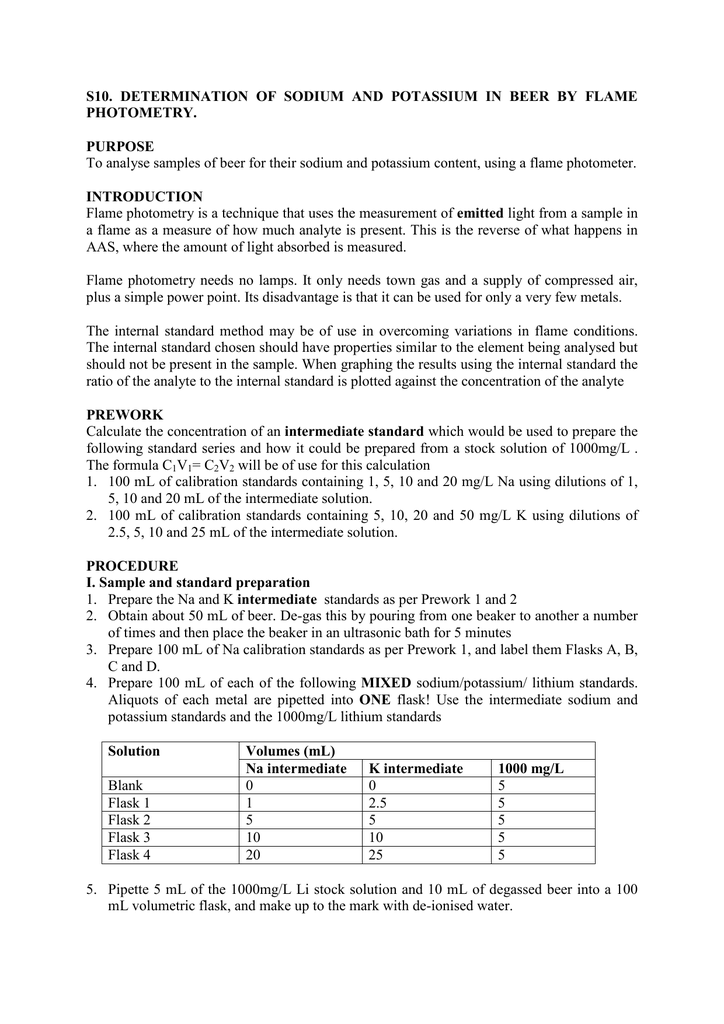
S10 Determination Of Sodium And Photometry Purpose

Pdf An Experimental Demonstration Of A Multi Element Flame Photometer Determination Of Salt Concentration In Soy Sauce Semantic Scholar
Comments
Post a Comment